Fire or Just Spark ?
The very origin of the fire that we all are aware of was discovered when the two stones rubbed together.
But what actually produce when the two stones rubbed together?
Was it a fire or just a spark?
Why those sparks couldn't persist for longer time?
It means that apart from rubbing the stones and creating sparks, there had to be something which could make it persist!
So now let's enter into the zone where we can figure out that what actually the most fundamental mandatory and sufficient conditions to cause the effect called FIRE .


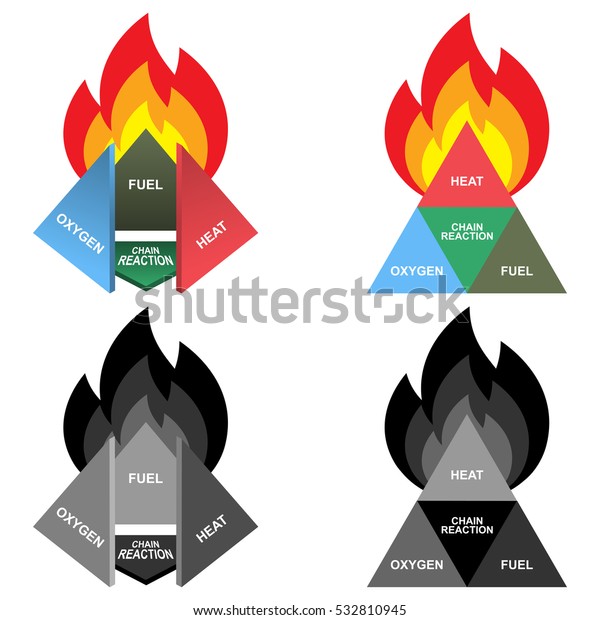
Fire Triangle -
Now if we rub the stones in a vacuum chamber, then what do you think will it cause any spark?
Obviously not because we know that Oxygen is the most fundamental thing that needs to be available where fire is present.
So, we got the first element of this fire triangle.
Now just try to have a look on combustion process. In all the combustion process oxygen is necessary element, but ultimately Oxygen burns what?
Yes, to the FUELS .
So here we got the second element of the fire triangle called Fuels.
It means that still we are lacking something .
Now again imagine a situation of the days in winter when we use to warm us by burning woods, over which we apply some fuels like kerosene or something. In this case, what we do when we have to burn it .
Yes, we get some sparks or any small source of fire (like from matchsticks). It means here we get our third element HEAT.
Now here we are, ready with our basic requirements of causing FIRE.

Now what do you think why the sparks that we got while rubbing the stone couldn't persist even after presence of oxygen?
So the one thing that's quite clear that although oxygen is utmost important for creating fire, but it is not everything , something else is left behind !Now just try to have a look on combustion process. In all the combustion process oxygen is necessary element, but ultimately Oxygen burns what?
Yes, to the FUELS .
So here we got the second element of the fire triangle called Fuels.
Now let's imagine a situation in which we apply oxygen gas over a fuel (for example petrol), will it catch fire?
Now again the answer is NO!It means that still we are lacking something .
Now again imagine a situation of the days in winter when we use to warm us by burning woods, over which we apply some fuels like kerosene or something. In this case, what we do when we have to burn it .
Yes, we get some sparks or any small source of fire (like from matchsticks). It means here we get our third element HEAT.
Now here we are, ready with our basic requirements of causing FIRE.
Now, after this fire triangle an addition was done, which converts this fire triangle into the Fire Tetrahedron.
Now if we look that wherever a fire is present there will be some combustion reaction takes place. So here we get our fourth element (although it's unavailability doesn't decide that whether a fire will occur or not, but helps in regulating it) i.e, Chemical Chain Reaction .
Now can we figure out some common phenomenon about which we might have never bothered so far?
How or Why water is capable of extinguishing the fire?
Actually blocking or displacing any single element from the Fire Tetrahedron can help in regulating the fire. So when we apply Water over the fire , it displaces the heat from the fire tetrahedron and resulting regulates the fire.
When we are talking about the fire caused due to electricity, then since water is a good conductor of electricity it increases the fire rather extinguishing it.
For fulfilling curiosity Distilled water in the form of mist can be used in extinguishing electrical fires .
How or why CO2 is capable of extinguishing the fire?
Actually, there are two reasons, but the main reason at which we should look at is that CO2 when apply over the fire region, then it displaces the oxygen from the fire tetrahedron and decreased amount of oxygen soon extinguishes the fire.
The other reason which not itself sufficient, but acts as a booster of the primary or the first reason is that when CO2 comes out from the fire extinguishers, then it is very cold and hence decreases one more element of the fire tetrahedron called Heat.
Now here we have dealt with producing and extinguishing the fire , but there are several other aspects as well as concerns too!
So, let's have a look from the interest's corner.
We know the answer of earlier one that when we approach any ignition source then different material responds differently. Like some catches fire violently (some even with exploding sound), some moderate and some very slowly (even in some cases several efforts have to be done) and some not at all.
Now the first thing is clear that flammable materials catch fire and non-flammable doesn't.
The answer is Volatility of materials, i.e, the higher the volatility of a material easier it will catch fire.
So, it's clear that why Petrol catches fire, more rapidly than diesel, kerosene etc.
Actually, when several experiments were done, then it was found that when the temperature of the fuel's ambient is reduced then it affects the fire's intensity.
The thing is that reducing the temperature causes reduction in the capability of the molecule of the liquid fuel to go into the vapor phase.
So, reducing the temperature causing trouble in catching the fire easily.
This reduction in temperature to a certain level leads to another aspect of burning Fire -

Actually the basic difference between the two is that at the flash point only a very small fraction of fuel vapor is present, which shows its presence when the ignition source is brought near to it but get's flicker out when takes away. But in the case of fire point, this point shows the minimum temperature that at which, when the fuel is made burn then it keeps burning for sometimes.
Now here we have dealt with producing and extinguishing the fire , but there are several other aspects as well as concerns too!
Like Does this Fire triangle works in every condition?
Does the fire generation depend also on the properties of fuel?
What do you think why we use coal, petrol, diesel, wood as a fuel why not anything else?
And among them why one is better than another?
Now the first thing is clear that flammable materials catch fire and non-flammable doesn't.
But what differs in the way of burning among the flammable materials?
The answer is Volatility of materials, i.e, the higher the volatility of a material easier it will catch fire.
So, it's clear that why Petrol catches fire, more rapidly than diesel, kerosene etc.
Now does this fire caught by any flammable materials depends on the other conditions too?
Actually, when several experiments were done, then it was found that when the temperature of the fuel's ambient is reduced then it affects the fire's intensity.
The thing is that reducing the temperature causes reduction in the capability of the molecule of the liquid fuel to go into the vapor phase.
So, reducing the temperature causing trouble in catching the fire easily.
This reduction in temperature to a certain level leads to another aspect of burning Fire -
Fire Point and Flash Point -
Fire Point -
Fire point is the lowest temperature at which a fuel is capable of burning when an ignition sources approaches it .
Flash Point -
Flash point is the lowest temperature at which that much vapor is present, which is capable of producing flash when the ignition source is approached to it.

Actually the basic difference between the two is that at the flash point only a very small fraction of fuel vapor is present, which shows its presence when the ignition source is brought near to it but get's flicker out when takes away. But in the case of fire point, this point shows the minimum temperature that at which, when the fuel is made burn then it keeps burning for sometimes.
Very informative blog till now
ReplyDeletethank you so much bhai for kept commenting on every article of mine!!!
DeleteQuiet informative blog,it covers almost every basic aspect of the process occuring which we generally ignore in our daily life...nice one 👌
ReplyDelete