Nitric Acid -
In case of inorganic chemistry, nitrogen is counted among those elements which has given the world, one of the most strong acid and i.e, Nitric acid.
Nitric acid has several other names like Aqua Fortis, spirit of niter, hydrogen nitrate etc. and some has significant reason like written below -
Aqua Fortis -
Nitric acid is also called aqua fortis because this is a latin word which means strong water .
Actually decontaminated Nitric acid looks like water but it is capable in dissolving the metals like silver.
The thing to be noted is that nitric acid is even capable in dissolving hard metals like iron, chromium, aluminium into it but when we get concentrated nitric acid for this purpose then rather making them dissolved, it started forming metal-oxides over the surface of these metals.
Actually decontaminated Nitric acid looks like water but it is capable in dissolving the metals like silver.
The thing to be noted is that nitric acid is even capable in dissolving hard metals like iron, chromium, aluminium into it but when we get concentrated nitric acid for this purpose then rather making them dissolved, it started forming metal-oxides over the surface of these metals.
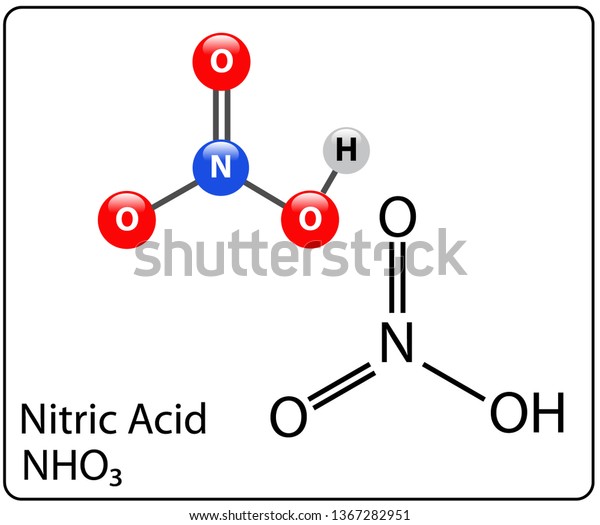
Nitric acid formula and structure -
HNO3 is the formula for nitric acid and hence it is also called hydrogen nitrate.

The charge shown on nitrogen and oxygen in the above figures are actually the formal charges which arises due to conversion of coordinate bond into covalent bond as per new rule.
Hybridization and Geometry -
The nitrogen atom being the central atom is sp2 hybridised and hence trigonal planar in geometry.


Acidity of HNO3 -
It is known that nitric acid is one of the most strong acid in inorganic chemistry but how this acidity is proved logically.
Actually there are several factors that governs the acidity of an acid but being straight forward, the stability of conjugate base tells you that how strong the acid is.
Resonating structures of nitric acid ( defining it's acidity ) -
The above shown canonical forms are the three possible resonating structures of nitric acid.
Here you may see that on releasing the H+ ion in aqueous solution, the nitrate ion carries one negative charge on the oxygen atom.
This negative charge is actually delocalized in between all the three oxygen atom present in the nitrate ion making it very stable conjugate base ( as the electronegativity of oxygen is capable in holding the negative charge ) hence the Nitric acid is so strong in nature.
Till now you have seen the basic info of nitric acid and it's acidity.
But now onwards you will get touched with those aspects of Nitric acid that are quite fascinating !

Fumes of Nitric acid -
Actually Nitric acid, on opening the cap of the glass bottle in which it is kept, produces strong vapours which we usually called fumes ( provided it should be pure nitric acid or of concentration greater then 86%).
The reason behind this is that at room temperature, the vapour pressure of nitric acid is larger than that of other volatile liquids like water. Also the vapours are visible in case of nitric acid.
Like for instance the vapour pressure of pure nitric acid at 20ºC is approx. 48 mmHg which is quite larger than that of water at the same temperature i.e, approx. 17 mmHg.
Actually the commercially used Nitric acid is having a concentration approx. 68% in water but for some specific applications, we use the high concentrated nitric acids (sometimes alongwith certain contaminants ).
Actually the commercially used Nitric acid is having a concentration approx. 68% in water but for some specific applications, we use the high concentrated nitric acids (sometimes alongwith certain contaminants ).
Now what would be the colour of this fume, will depend upon the concentration of certain contaminants.
White Fuming Nitric acid -
When the Nitric acid is not contaminated enough i.e, if it is present in a concentration, at least about 95 % ( provided that the concentration of NO2 due to somehow contamination should not be greater than 0.5 % ) then we get the dense white fumes of nitric acid.
Actually this concentration of Nitric acid is not for general use. We use it as an oxidizer alongwith kerosene and hydrazine in Rocket fuels.
Red Fuming Nitric acid -
This form of Nitric acid is also produced for using it as an oxidizer in rocket propellants.
When we add dinitogen tetroxide (N2O4) which is colourless, in pure nitric acid in such a way that the composition consist around 84% of HNO3, 1-2% of H2O and around 13% of N2O4 then the N2O4 gets break down partially to form NO2 ( which is brown in colour ).
This NO2 gets dissolved in the solution until it becomes saturated and this gas gets evaporates off in the atmosphere giving dense fumes of NO2 which is dark red or bown in colour.
This gas gives suffocating odour so it is not recommended to follow this procedure in a normal way in laboratories.
Now let's go with the two uses of Nitric acid out of the box .
Manufacturing of fertilizers, plastics and dyes are the straight uses of Nitric acid.
Apart from them, there are certain chemicals which are manufactured with the help of Nitric acid but they are quite different from the above mentioned one !
Dynamite -

You might have heard about the explosives like dynamite which is used by the mining industries for extracting ores of the several minerals found in earth's crust.
Almost whatever the products that we use like utensils, electronic gadgets, wires etc. they all contain several minerals like metals, semi- metals, non-metals.
So it shouldn't be the case to make you realize that how much beneficial these mining industries are.
So definitely Dynamite, on making this process easy is also play a very significant role in this.
Almost whatever the products that we use like utensils, electronic gadgets, wires etc. they all contain several minerals like metals, semi- metals, non-metals.
So it shouldn't be the case to make you realize that how much beneficial these mining industries are.
So definitely Dynamite, on making this process easy is also play a very significant role in this.
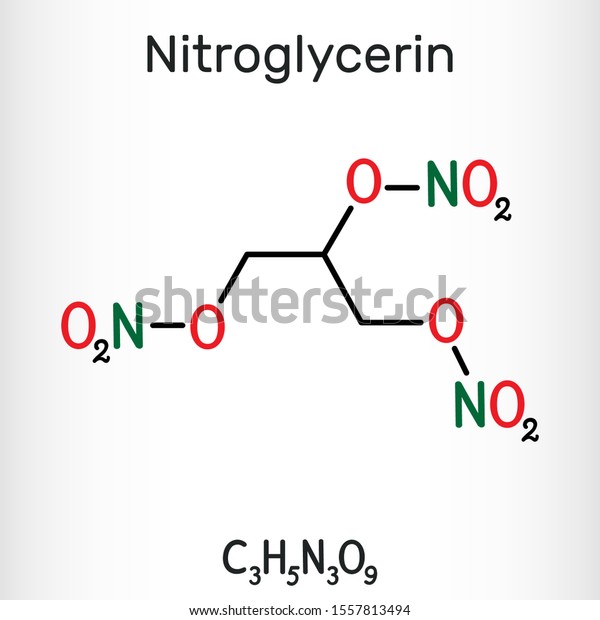
Here you will get encounter with the active ingredient of Dynamite and i.e, Nitroglycerin .
Nitroglycerin ( NG ) -

Nitroglycerin (NG) is mainly an explosive which is the active ingredient in dynamite. It is basically a dense, oily and colourless liquid and how is produced is the main concern with how it is relevant with the ongoing chemical i.e, NITRIC ACID.
So as you got to know the two types of fuming nitric acids, white and red respectively.
The main use that you have seen is that both the forms are used as an oxidizer in rocket propellants.
So as you got to know the two types of fuming nitric acids, white and red respectively.
The main use that you have seen is that both the forms are used as an oxidizer in rocket propellants.
But here let's see the other application of White Fuming Nitric acid.
Actually this Nitroglycerin ( an explosive ) is produced with the help of two chemicals.
The one is nitrating glycerol and the other one is White Fuming Nitric acid !
Actually White Fuming Nitric acid is the purest and the most expensive form of Nitric acid hence it is not easily available in the market and synthesized for the specific purposes like as an oxidizer in the rocket propellants and in manufacturing Nitroglycerine.
Talking about the detonation part of the Nitroglycerin then unlike other normal explosives it results in producing supersonic waves as soon as it catches fire.
The other advantageous part of this explosive is that it doesn't produce any solid carbon ( Like Shoot ). So it can be said that this is one of the smokeless explosive along with the production of a huge amount of energy as well as gases.
Actually White Fuming Nitric acid is the purest and the most expensive form of Nitric acid hence it is not easily available in the market and synthesized for the specific purposes like as an oxidizer in the rocket propellants and in manufacturing Nitroglycerine.
Detonation of NG -
Actually Nitroglycerin is a very unstable compound hence even a small friction or jerk leads to a violent explosion because being an unstable compound this always ready to rearrange itself in the simpler stable molecules like N2 and CO.
Talking about the detonation part of the Nitroglycerin then unlike other normal explosives it results in producing supersonic waves as soon as it catches fire.
The other advantageous part of this explosive is that it doesn't produce any solid carbon ( Like Shoot ). So it can be said that this is one of the smokeless explosive along with the production of a huge amount of energy as well as gases.

Bhaisahab, Gyaan.......😫
ReplyDelete😂😂 Sorry that was a joke 😄
Btw Good content 👍✌️❤️❤️❤️.
are bhai bhai, thanx a lot !!
Deletethanx bhai!
ReplyDelete