Hydrochloric Acid -

It's pretty sure that you get exhausted while dealing with the casual physical and chemical properties of the chemicals. So here you will get encountered with the usual chemicals in unusual ways that you kept dealing with !

Actually these acids are very strong and corrosive in nature and at certain dilution they are used for several purposes like -
Sulfuric acid is mainly used in the production of other mineral acids as well as used in producing chemicals like synthetic detergents, dyes, pigments, several varieties of drugs etc.
Nitric acid is used to manufacture Ammonium Nitrate which is further used in making fertilizers. It is also used in the production of explosive chemicals like Nitroglycerin and TNT.
Hydrochloric acid is mainly used in batteries, firecrackers and also in the lab preparation of table salt ( NaCl ).
So how the use of this HCl dominates over the other mineral acids ?
Gastric Juice -
Organic Nature of Inorganic HCl -
Although the role of the other mineral acids like Nitric acid, Sulfuric acid, Boric acid etc. is appreciable for humankind but how HCl plays role for human life is something that makes it dominating over the other mineral acids.
Being used in Gastric Juice, HCl is organically involved in staying us alive !
It is not mere a simple acid that involves in digesting the food that we eat but it is something that allows us to eat whatever we chose !
And because of only this, it makes us capable to discover uncountable no. of dishes across the religion, culture, society, state, country and the world on every single day !
Chemistry Of Gastric Juice -
Although Gastric Juice contains other chemicals along with HCl like water, electrolytes, several enzymes etc. but the role that HCl plays being used in Gastric juice is something that is so important for the human internal system.
Actually whatever we eat is a source of nutrient like carbs, proteins, fats etc. And on a broad sense, it is the role of the internal organs to extract these nutrients into simplest form and help them to get transported into the bloodstream .
How HCl helps into this ?
Actually the protein that we eat, needed to be broken down into their constituent amino acids and to carry out this process an enzyme called Pepsin is required.
But the problem is that in the stomach pepsin is present in it's inactive form i.e, pepsinogen.
This HCl which is present in the gastric juice helps in converting this enzyme called pepsinogen into it's active form i.e, Pepsin.
Along with this, HCl also helps in killing out the harmful pathogens like viruses which brought into the stomach by the food that we eat.
Other aspects of HCl -
Hydrochloric acid formula -
You might be reacting weird that what's the big deal with the formula of hydrochloric acid, as it is simple as HCl in which hydrogen and chlorine atoms get bonded with a single covalent bond.
But it is actually the root of all the interesting results or applications of HCl which you see in the real world !
Bond in HCl -
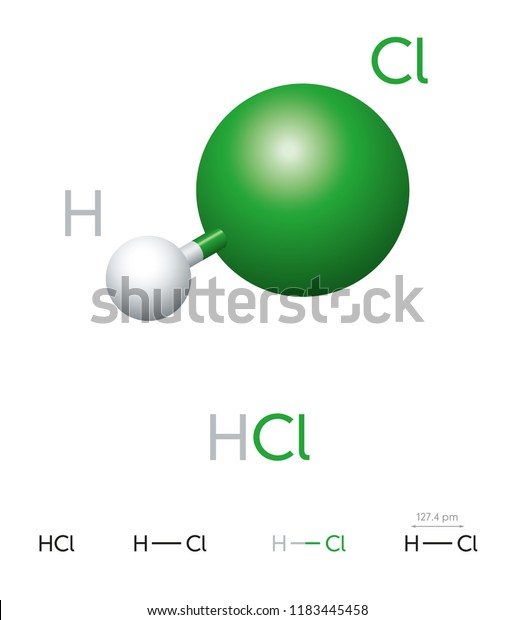
The first thing about the bond between the hydrogen and chlorine atom in this molecule is that, it is a single covalent bond which is highly polar in nature.
Being the third most electronegative element of the periodic table, Chlorine always tends to form molecules involving electrovalent and polar covalent bonds .
In case of HCl, the electronegativities of Hydrogen and Chlorine are 2.2 and 3.16 respectively. The difference between this is significant enough to make it behaves like a strong electrolyte and here what it is.
Making it more shocking for you -
Yet you might not be feeling so strange about this, but lets take the example of water in which same hydrogen is present which is present in HCl and one another element i.e, Oxygen atom which is even more electronegative than Cl having electronegativity around 3.44.
So here the thing is clear to you that the difference in electronegativities of bonded atoms in H2O is more as that in HCl, yet water is a very weak electrolyte.
So here the thing is clear to you that the difference in electronegativities of bonded atoms in H2O is more as that in HCl, yet water is a very weak electrolyte.
Comments
Post a Comment
Please don't enter any spam link in the comment box.